What is CRISPR-Cas9?
Image credit: Shutterstock
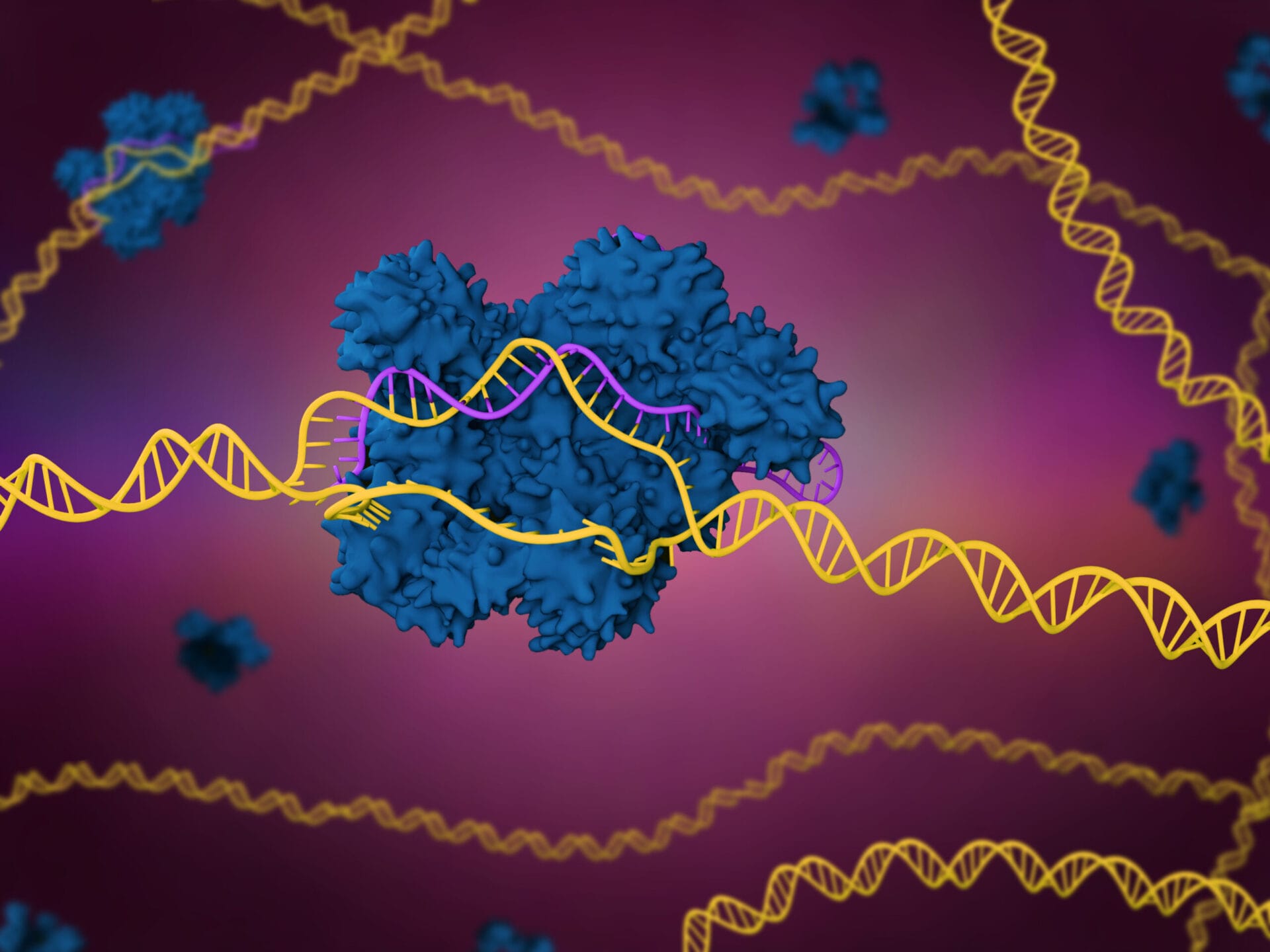
CRISPR-Cas9 is a genome editing tool that is creating a buzz in the science world. It is faster, cheaper and more accurate than previous techniques of editing DNA and has a wide range of potential applications.
- CRISPR-Cas9 is a unique technology that enables geneticists and medical researchers to edit parts of the genome by removing, adding or altering sections of the DNA sequence.
- It is currently the simplest, most versatile and precise method of genetic manipulation and is therefore causing a buzz in the science world.
How does CRISPR-Cas9 work?
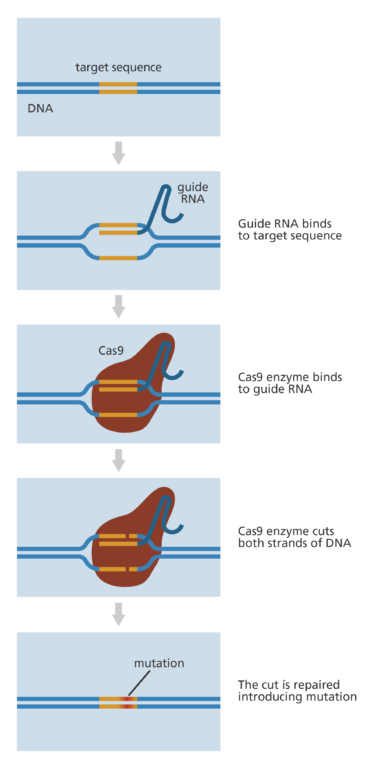
- The CRISPR-Cas9 system consists of two key molecules that introduce a change (mutation) into the DNA. These are:
- an enzyme called Cas9. This acts as a pair of ‘molecular scissors’ that can cut the two strands of DNA at a specific location in the genome so that bits of DNA can then be added or removed.
- a piece of RNA called guide RNA (gRNA). This consists of a small piece of pre-designed RNA sequence (about 20 bases long) located within a longer RNA scaffold. The scaffold part binds to DNA and the pre-designed sequence ‘guides’ Cas9 to the right part of the genome. This makes sure that the Cas9 enzyme cuts at the right point in the genome.
- The guide RNA is designed to find and bind to a specific sequence in the DNA. The guide RNA has RNA bases that are complementary to those of the target DNA sequence in the genome. This means that, at least in theory, the guide RNA will only bind to the target sequence and no other regions of the genome.
- The Cas9 follows the guide RNA to the same location in the DNA sequence and makes a cut across both strands of the DNA.
- At this stage the cell recognises that the DNA is damaged and tries to repair it.
- Scientists can use the DNA repair machinery to introduce changes to one or more genes in the genome of a cell of interest.
How was it developed?
- Some bacteria have a similar, built-in, gene editing system to the CRISPR-Cas9 system that they use to respond to invading pathogens like viruses, much like an immune system.
- Using CRISPR the bacteria snip out parts of the virus DNA and keep a bit of it behind to help them recognise and defend against the virus next time it attacks.
- Scientists adapted this system so that it could be used in other cells from animals, including mice and humans.
What other techniques are there for altering genes?
- Over the years scientists have learned about genetics and gene function by studying the effects of changes in DNA.
- If you can create a change in a gene, either in a cell line or a whole organism, it is possible to then study the effect of that change to understand what the function of that gene is.
- For a long time geneticists used chemicals or radiation to cause mutations. However, they had no way of controlling where in the genome the mutation would occur.
- For several years scientists have been using ‘gene targeting’ to introduce changes in specific places in the genome, by removing or adding either whole genes or single bases.
- Traditional gene targeting has been very valuable for studying genes and genetics, however it takes a long time to create a mutation and is fairly expensive.
- Several ‘gene editing’ technologies have recently been developed to improve gene targeting methods, including CRISPR-Cas systems, transcription activator-like effector nucleases (TALENs) and zinc-finger nucleases (ZFNs).
- The CRISPR-Cas9 system currently stands out as the fastest, cheapest and most reliable system for ‘editing’ genes.
What are the applications and implications?
- CRISPR-Cas9 has a lot of potential as a tool for treating a range of medical conditions that have a genetic component, including cancer, hepatitis B or even high cholesterol.
- Many of the proposed applications involve editing the genomes of somatic (non-reproductive) cells but there has been a lot of interest in and debate about the potential to edit germline (reproductive) cells.
- Because any changes made in germline cells will be passed on from generation to generation it has important ethical implications.
- Carrying out gene editing in germline cells is currently illegal in the UK and most other countries.
- By contrast, the use of CRISPR-Cas9 and other gene editing technologies in somatic cells is uncontroversial. Indeed they have already been used to treat human disease on a small number of exceptional and/or life-threatening cases.
What’s the future of CRISPR-Cas9?
- It is likely to be many years before CRISPR-Cas9 is used routinely in humans.
- Much research is still focusing on its use in animal models or isolated human cells, with the aim to eventually use the technology to routinely treat diseases in humans.
- There is a lot of work focusing on eliminating ‘off-target’ effects, where the CRISPR-Cas9 system cuts at a different gene to the one that was intended to be edited.
Better targeting of CRISPR-Cas9
- In most cases the guide RNA consists of a specific sequence of 20 bases. These are complementary to the target sequence in the gene to be edited. However, not all 20 bases need to match for the guide RNA to be able to bind.
- The problem with this is that a sequence with, for example, 19 of the 20 complementary bases may exist somewhere completely different in the genome. This means there is potential for the guide RNA to bind there instead of or as well as at the target sequence.
- The Cas9 enzyme will then cut at the wrong site and end up introducing a mutation in the wrong location. While this mutation may not matter at all to the individual, it could affect a crucial gene or another important part of the genome.
- Scientists are keen to find a way to ensure that the CRISPR-Cas9 binds and cuts accurately. Two ways this may be achieved are through:
- the design of better, more specific guide RNAs using our knowledge of the DNA sequence of the genome and the 'off-target' behaviour of different versions of the Cas9-gRNA complex.
- the use of a Cas9 enzyme that will only cut a single strand of the target DNA rather than the double strand. This means that two Cas9 enzymes and two guide RNAs have to be in the same place for the cut to be made. This reduces the probability of the cut being made in the wrong place.