What is a clinical trial?
Image credit: Shutterstock

A clinical trial is a research investigation when a new treatment is first given to humans to establish if it is safe and effective at treating the disease.
- Clinical trials are research investigations that specifically involve human volunteers.
- They are carried out once a treatment has been shown to be safe and effective in pre-clinical studies.
- They are usually the final step before a drug can start to be approved for use in standard treatments.
What is a clinical trial?
- Clinical trials, also known as clinical development or clinical studies, are research investigations set up to establish the effectiveness and safety of new treatments.
- For example, trials can be used to test new drugs, new drug combinations, other forms of treatments such as surgery, and preventative medicines such as vaccines.
- Clinical trials are only carried out once a treatment has been proved successful in the laboratory using cell or tissue culture systems or in model organisms (Stage 3 of drug development).
- As part of the whole drug development process, carefully regulated clinical trials are currently the most effective way to establish which new treatments are beneficial for human health.
How does a clinical trial work?
- Clinical trials specifically involve human volunteers. Participants in clinical trials are generally divided into two or more groups.
- Most trials are randomised, which means that the participants are randomly allocated to their treatment groups. This ensures people do not know if they are being given the drug under investigation or not.
- In some cases, even the scientists and doctors involved in the trial don’t know which patient is part of which treatment group until the trial is over.
- This is called a ‘double-blinded experiment’ and ensures that neither the patients nor investigators are biasing the outcome of the clinical trial by their prior expectations.
- Randomisation also helps to minimise the differences between the groups by equally distributing people with particular characteristics.
What are the different treatment groups involved in a clinical trial?
The treatment group
- Patients in the treatment group are given the new therapy that is being tested in the trial.
The control group
- One of the groups is always a control group. This is the standard against which the effectiveness of the new treatment is judged.
- In some cases, the control group is the current standard treatment commonly used for the condition under investigation. For example, if the trial is testing a new combination of drugs, the control group might receive a single one of these drugs.
- In some cases, the participants may be given no treatment at all, known as placebo.
Why do trials use a placebo?
- If the control group is not given an active drug, they are often given a placebo.
- This is usually a treatment that visibly resembles the experimental drug (such as a tablet that looks the same) but has no active compounds, so will not have an effect on the individual.
- Placebos are important because they allow clinical trials to be carried out under double-blind conditions. This removes any bias and helps to produce fair and objective results.
- This is important because if the patient or the researcher knows which group they are in, it can change the way the patient behaves and affect the results of the study.
Phases in a clinical trial
- For a drug to be approved for use in patients, it must be just as safe and more effective as the current standard treatment.
- There are usually four key phases in a clinical trial, but sometimes there can be five.
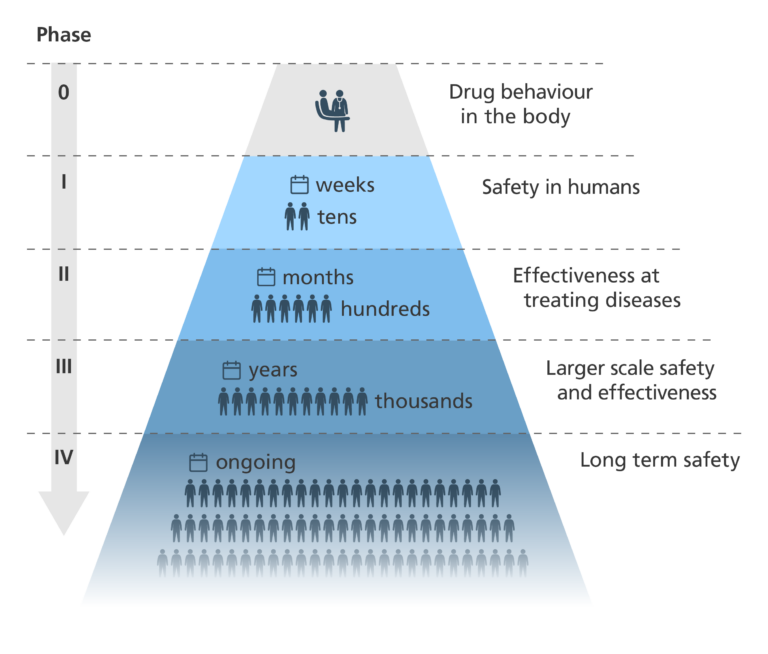
Phase 0
- Trials don’t always involve a Phase 0 stage – most jump straight to Phase I.
- If Phase 0 is run, a very small group of people are given a very small dose of a drug.
- Phase 0 looks to see:
- if the drug reaches its target – for example, a tumour.
- how the drug behaves in the body.
- how target cells in the body respond to the drug – for example, how cancer cells respond.
- The main aim of Phase 0 is to speed up the development of promising new drugs. Testing some drugs in very small doses in humans gives a clearer idea of drug-target interactions than doing the same in model organisms.
Phase I
- Phase I studies involve a small group of participants. For many new treatments, the participants are healthy volunteers. For some conditions, such as cancer, the participants are people with the condition.
- Phase I of a clinical trial is designed to evaluate the safety of the new treatment and whether it causes any side effects. In the case of new drugs, a safe dose range is worked out.
- This phase usually lasts a few months.
Phase II
- Phase II studies involve a larger group of people than in Phase I. In Phase II, all participants have the condition being targeted by the therapy.
- This phase is designed to test how effective the new treatment is at treating the condition.
- To ensure objectivity, these studies are usually randomised and double-blinded.
- These studies often take around two years.
Phase III
- Phase III trials are similar to Phase II trials, but on a much larger scale, involving hundreds or even thousands of participants.
- They collect large amounts of information about the treatment’s safety and effectiveness.
- Once these trials are completed, the treatment may be approved for general use.
- This phase may take several years.
Phase IV
- After a treatment is approved for general use in Phase III, it enters Phase IV post-marketing trials to evaluate its long-term safety.
- Phase IV uses feedback from doctors and patients and is used to find out more about the side effects and safety of the drug.
- It can improve our understanding of the long-term risks and benefits of the treatment.
- Phase IV also shows how well the new treatment works when it is used more widely.
Covid-19 vaccine clinical trials
- The traditional clinical trial process can take years for a drug to be used in the general population. Since the process is expensive and not all drugs are proven to be safe and effective, it usually follows the linear Phase 0 to Phase IV sequence.
- However, during the pandemic, patients needed new treatments and populations needed new vaccines in a much shorter timeframe than the traditional process would enable.
- New vaccines were developed in just one year. This came about because:
- Different stages of development and production were carried out at the same time.
- New collaborative approaches meant scientists and regulatory bodies were working together around the world.
- Increased funding meant many promising techniques and technologies could be tested.
- Manufacture capacity was dramatically increased so that the vaccine could be produced at unprecedented scale.