C elegans: the early worm gets the sequence
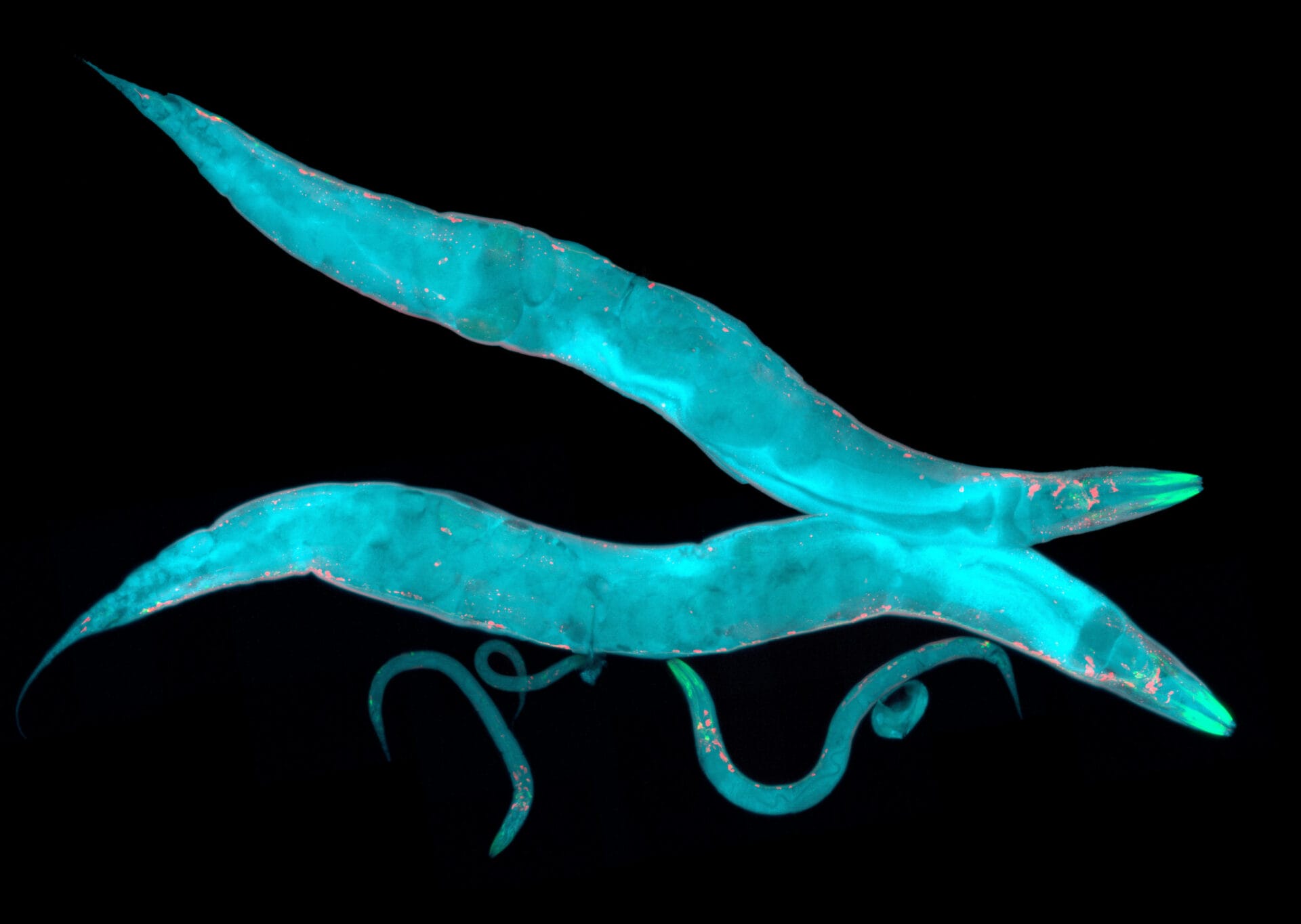
Since the emergence of the nematode worm as a model organism in the 1960s, it’s helped to unlock important information and unravel mysteries of biology.
- The emergence of the soil-dwelling nematode worm – Caenorhabditis elegans – as a model organism brought much excitement to the field genetics.
- It has since been used to understand a range of biological processes and human conditions, including cancer and dementia. Read more on our fact page.
Key terms
Model organism
A species that has been widely studied in biology, usually because it is easy to maintain and breed in a laboratory setting and has particular experimental advantages.
DNA
(deoxyribonucleic acid) A molecule that carries the genetic information necessary to build and maintain an organism.
Genome
The complete set of genetic instructions required to build and maintain an organism.
DNA sequencing
The process of determining the order of bases in a section of DNA.
From the beginning
In the 1960s, Sydney Brenner was a young scientist from South Africa who had worked with Francis Crick looking at the function of DNA. For him, the future of molecular biology was clear: we needed to investigate development and the nervous system. But first, he needed to find an appropriate model organism on which to base his work.
Caenorhabditis elegans (C elegans) is a soil-dwelling, bacteria-eating nematode worm about 1 mm in length. At the time, it was unknown in the world of biological research. However, its characteristics fitted Sydney’s experimental criteria very neatly: it has simple and rapid genetics, it grows quickly and can be kept in the lab conveniently, and is small, so its anatomy and development can be examined easily.
The worm is also much simpler than humans. For example, it doesn’t have bones, a heart or a circulatory system. But despite this simplicity, many of the genes, cellular processes and signals involved in C elegans development are also found in more complex organisms.
With the goal of making the link between genes and behaviour, Sydney Brenner began using the C elegans as a model organism in his lab in Cambridge, UK. He started by making worms with mutated genes, then worked backwards to find specific genes responsible for behaviour, the nervous system and muscle movement.
The new C elegans model organism brought much excitement to the field. Consequently, worm research expanded considerably during the late 1960s and 1970s.
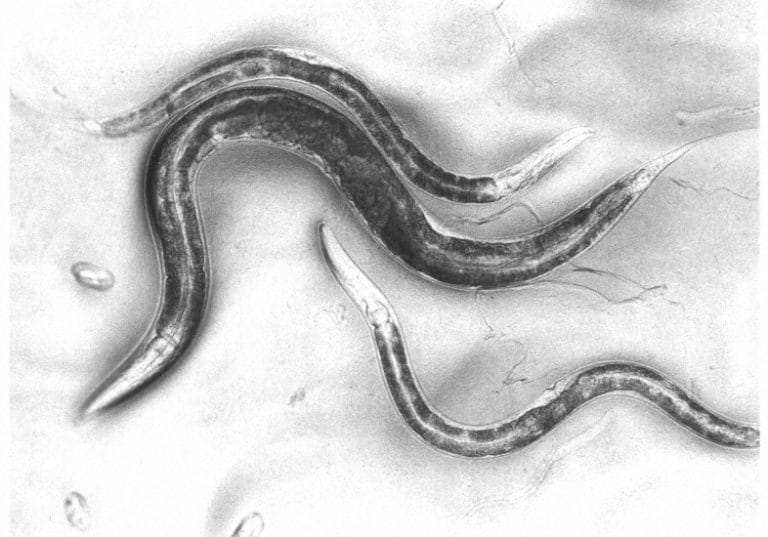
The nematode worm has simple and rapid genetics, grows quickly and can be kept in the lab conveniently – making it an optimal model organism.
Revealing ‘programmed cell death’
John Sulston joined Sydney’s group in 1969 and spent the next decade working out how C elegans develops from a single cell to an adult. Since the worm is transparent, the fine detail of its cells can be seen under a microscope, making it possible to follow the division of cells as the worm develops and grows. The adult C elegans contains about 1,000 cells, and it took about 10 years for scientists to completely map its development.
This mapping project revealed an entirely new biological phenomenon. Some of the initial cells never make it into the adult worm and are instead programmed to die during development. The process was given the name ‘programmed cell death’ or ‘apoptosis’.
Programmed cell death has a fundamental role in human development – such as the programmed death of cells in our hands to create fingers. Its discovery also helped us to understand more about different human diseases. For example, during the development of AIDs and in heart attacks, lots of cells are lost through excessive cell death. In other conditions, such as autoimmune conditions and cancer, cells survive that are normally destined to die.

Since C elegans is transparent, the fine detail of its cells can be seen under a microscope.
Understanding cell death in neurodegeneration
Robert Horvitz, an American biologist, spent a large portion of his working life exploring apoptosis, using C. elegans as an experimental model. He was particularly interested in the role of apoptosis in human neurodegenerative disease.
Robert showed that cell death is often an active process and that many of the genes that control the death of neurons in C elegans have counterparts in the human brain. His discoveries paved the way for a deeper understanding of neurodegenerative conditions, such as Alzheimer’s disease, Parkinson’s disease and Huntington's disease.
In October 2002, Sydney Brenner, John Sulston and Robert Horvitz jointly received the Nobel Prize in Physiology or Medicine for their work studying organ development and programmed cell death – something that would have been possible without the help of the humble nematode worm.
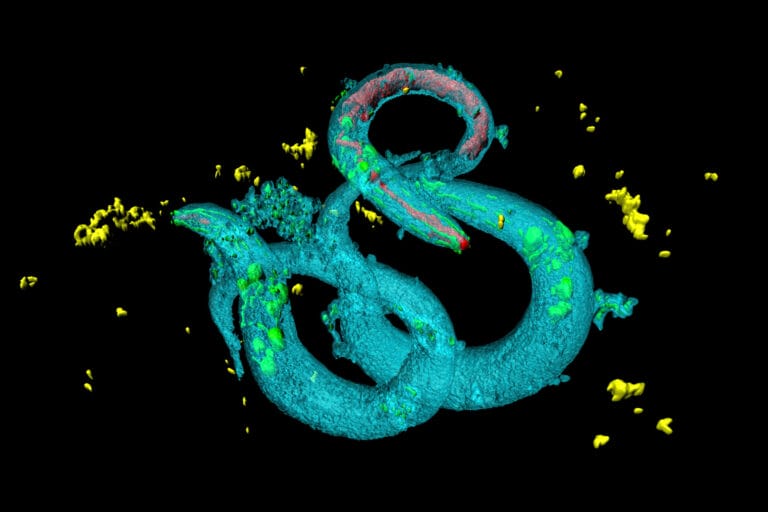

Mapping the development of the worm revealed an entirely new biological phenomenon: programmed cell death.
Mapping the genome of C elegans
By the early 1980s, many mutant C elegans strains had been created, with genes or sections of DNA changed to influence certain characteristics. It became clear that researchers needed to find the genes responsible for the mutants to understand what was going on at the genetic level. The next logical step was to map the genome of the worm to highlight where each gene is located in the genome.
Gene or genome mapping is when scientists identify the locations of different genes on specific chromosomes – a bit like pointing out key landmarks on a geographical map. The advantage of this technique is that you know where genes are in relation to each other.
John Sulston mapped out the C elegans genome, along with his colleagues Alan Coulson and Bob Waterston. The map consisted of multiple overlapping fragments of DNA, arranged in the correct order.
This marked the beginning of the study of animal genomes. The next step would be to work out the exact order of the letters in the C. elegans genome.

The next logical step was to map the genome of the worm to highlight where each gene is located in the genome.
Sequencing the C elegans genome
The worm sequencing project began in 1990 and, working with the new automated DNA sequencing machines, met its target of sequencing the first three million bases in three years.
This was only 3% of the whole worm genome, but it was an important proof-of-principle for the Human Genome Project. It showed that the DNA sequencing technology was scalable and that with more money, more people, and more machines, the three billion bases of DNA in the human genome could be tackled.
In 1998, the sequence of the entire C elegans genome was published – the first animal to have its genome completely sequenced. The whole genome enabled a deeper understanding of the worm’s 20,500 genes, including identifying whole gene families and the examination of patterns of gene expression.

In 1998, the sequence of the entire C elegans genome was published – the first animal to have its genome completely sequenced.
Ongoing research
The worm is now a classic model organism that has enabled huge leaps forward in scientific discovery – and we now know that more than 75% of genes that cause diseases in humans have counterparts in C elegans. Understanding the genetics of C elegans has allowed scientists to better understand human genetics, conditions and diseases.
Ageing
Like humans, C elegans exhibits many physical and behavioural traits that decline with age, making the worm a useful model to study the ageing process. For example, the ‘insulin/insulin-like growth factor-1 (IGF-1) signalling pathway’, which regulates ageing in worms, is also present in humans and other mammals.
The worm has also taught us more about telomeres – the protective caps that sit on the ends of chromosomes, a bit like a shoelace tip. For a long time, scientists thought that the length of these telomeres indicated how long the organism lived. However, studies in C elegans have shown that telomere length is independent of ageing: worms with short telomeres live for just as long as worms with long telomeres. This was also found to be true in humans.
Dementia
Understanding the ageing process in C elegans has also helped to provide important clues about age-related conditions, such as cardiovascular disease and different types of dementia, including Alzheimer’s disease.
For example, researchers believe that Alzheimer’s disease causes dementia when certain proteins misfold and begin accumulating, leading to damaged brain cells. Scientists have been able to recreate this phenomenon in C elegans – the protein folds incorrectly and accumulates in the worm’s nerve cells, leading to behavioural abnormalities and the nerve cells dying.
Taking this a step further, they’ve shown that certain drugs can prevent this misfolding and accumulation in the worm – which could lead to a way to prevent Alzheimer’s disease.
Using C elegans to study genes linked to neurodegeneration is also providing insight into conditions like motor neuron disease, which could lead to much needed new treatments.
Cancer
Understanding cancer genes is crucial for developing treatments. Although C elegans don’t develop tumours, we now know that the worm has more genes related to human cancer genes than any other model organism. This has provided important clues about how the genes function and lead to the development of the condition.
For example, programmed cell death, or apoptosis, usually helps to prevent cancer in humans, by eliminating cells that are behaving abnormally. Understanding the process in C elegans has shown us how it works in humans.
Another process that is important in the behaviour of cells is Wnt (pronounced “wint”) signalling – when it is faulty in humans, this can lead to the development of cancer. Understanding this process in the worm has shed important light on how it functions in humans, leading to experimental drugs that could help to treat different types of cancer.
WormBase
WormBase is an online database that stores information about the biology and genome of C elegans and other related nematodes. It’s used as a central resource for scientists to find and share experimental data and information.
The resource is highly detailed and regularly updated, containing genetic maps of different species, how different mutations affect characteristics, and details about worm biology – which can be used to better understand human conditions.